Hope for better heart disease treatments

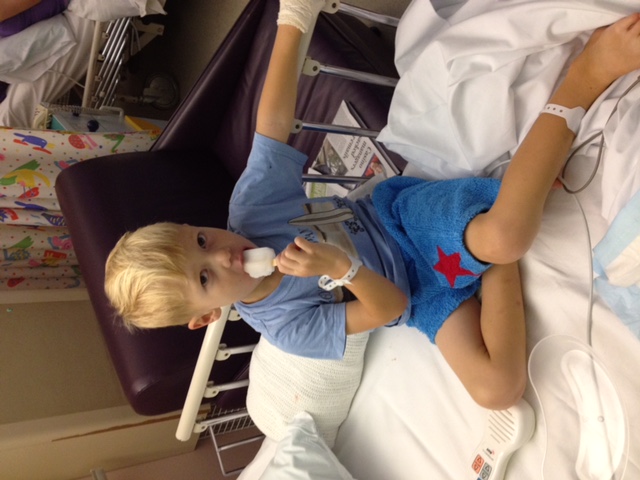
From the moment William Pearce (pictured below) had his first cardiac arrest, his life has largely been in the hands of others.
William was diagnosed as a newborn with hypertrophic cardiomyopathy, a genetic condition where the heart muscle becomes thickened. This condition makes it harder for the heart to pump blood and can cause shortness of breath, chest pain and cardiac arrests. Due to the condition, William was dependent on having those around him know CPR.
Mum Nicala Pearce said with his cardiac episodes occurring every few months, the family always had to brace themselves for the prospect he wouldn’t make it home one day.
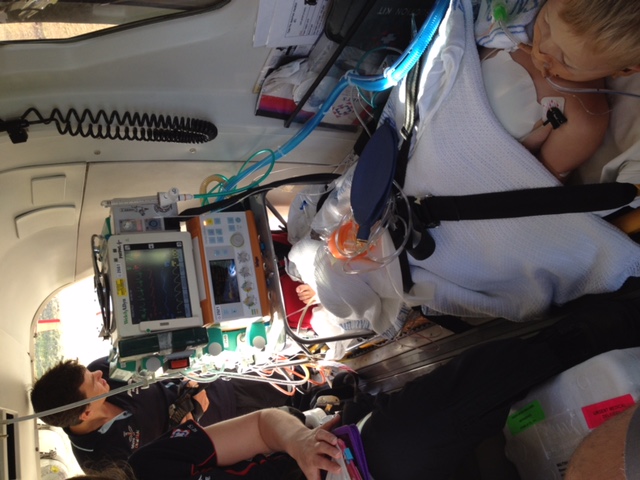
The constant dread began when William was just four and went into his first cardiac arrest at home.
“I found him collapsed on the bedroom floor, unresponsive and I had to do CPR while my other son called for help,” Nicala said. "Once we arrived at the hospital, he was put in a week long coma and after weeks of treatment he was able to come home.”
Following the attack, William had to be implanted with a defibrillator to monitor and shock his heart if he went into cardiac arrest. A device he would have for the next eight years.
“He just had constant cardiac episodes and the only way for him to survive these attacks was to be shocked internally and externally and then receive CPR,” Nicala said. "These cardiac events also occurred at school where the teachers would have to save his life. When we would send him off to school we always thought it could be the last time. He is extremely lucky to still be with us today.”
Nicala (pictured below with family) said the family had never heard of the genetic heart condition before William’s diagnosis.
“We knew there was a family history of heart issues but we didn’t realise there was genetic connection,” she said. "We were just frustrated and devastated to get the news because things could have been so different had we known.”
Nicala said due to his condition William couldn’t play sport or do any form of exercise.
“He wasn’t allowed to run or jump, couldn’t walk upstairs and lived a very sedentary life,” she said. "It was like living with an invisible disability.”
But everything changed when William, now aged 16, received a heart transplant four years ago.
“William is finally living and enjoying life,” Nicala said. "He is playing footy, learning how to surf and going to the gym. He is embracing life to the full.”

Nicala said new research by Murdoch Children’s Research Institute that has discovered how a gene that increases the risk of developing genetic heart disease works, paving the way for new treatments, would come as a huge relief to other families affected by the condition.
“With the condition there are limits to what doctors can do,” she said. "As a mum, you are always trying to find the answers to make things better but there was nothing we could do for William. This research will give so many families hope for the first time that new treatments could finally be discovered.”
Heart disease is the leading cause of death worldwide. Patients with genetic cardiomyopathy, affecting about 35 million people, have a greater risk of heart failure and death.
Dr James McNamara said often the disease was caused by genetic mutations that impacted heart muscle function. The gene, ALPK3, that controls the heart’s capacity to beat normally had been shown to increase cardiomyopathy risk when mutated, he said.
“Mutations in this gene can cause very severe and sometimes fatal cardiomyopathy in children,” he said. But it has been unknown what ALPK3 does in the heart and how its mutation causes disease.
“Our research is the first to show how ALPK3 directly controls the function of contractile proteins in the heart that drive normal pumping. We found that ALPK3 links these contractile proteins to quality control systems and that its mutation hinders this link, causing a build-up of damaged proteins. This impairs the heart’s ability to pump blood to the rest of the body, causing breathlessness, swollen legs and feet and extreme fatigue and if left untreated can lead to heart failure.”
Murdoch Children’s Professor Enzo Porrello said the findings could lead to new drug discoveries to treat cardiomyopathy.
With limited treatment options for patients, new targeted therapies are desperately needed,” he said. "But armed with a greater understanding of how this gene works and by engineering stem cells in the lab to model genetic cardiomyopathy we can screen for new drugs and identify disease mechanisms. This could lead to new targeted treatments that restore heart function.”
Read more on the study.